Free Shipping in the U.S. for orders over $1000. Shop Now>>

Formalin-fixed, paraffin-embedded human adrenalgland stained with IL6RB / CD130 Mouse Monoclonal Antibody (IL6ST/4101). HIER: Tris/EDTA, pH9.0, 45min. 2°C: HRP-polymer, 30min. DAB, 5min.
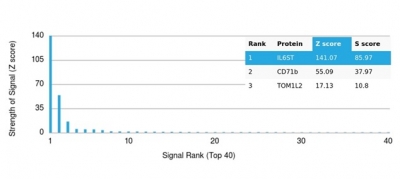
Analysis of Protein Array containing more than 19,000 full-length human proteins using IL6RB / IL6ST Monospecific Mouse Monoclonal Antibody (IL6ST/4101). Z- and S- Score: The Z-score represents the strength of a signal that a monoclonal antibody (MAb) (in combination with a fluorescently-tagged anti-IgG secondary antibody) produces when binding to a particular protein on the HuProtTM array. Z-scores are described in units of standard deviations (SD's) above the mean value of all signals generated on that array. If targets on HuProtTM are arranged in descending order of the Z-score, the S-score is the difference (also in units of SD's) between the Z-score. S-score therefore represents the relative target specificity of a MAb to its intended target. A MAb is considered to be specific to its intended target, if the MAb has an S-score of at least 2.5. For example, if a MAb binds to protein X with a Z-score of 43 and to protein Y with a Z-score of 14, then the S-score for the binding of that MAb to protein X is equal to 29.
Interleukin-5, or IL-5, was originally discovered as a soluble T cell-derived factor, called T cell-replacing factor (TRF), that induced T cell-depleted activated B cells to secrete immunoglobulin. Native IL-5 is a disulfide-linked homodimer. IL-5 is initially synthesized as a precursor with a 19 amino acid signal peptide which is cleaved to form a 112 amino acid mature protein. Murine and human IL-5 exhibit 70% sequence identity at the amino acid level. IL-5 exerts its biological activity through the IL-5 receptor (IL-5R), which is composed of at least two chains: an chain that binds IL-5 with low affinity and a chain that does not bind IL-5, but together with the IL-5 a chain, constitutes the high affinity IL-5 receptor. The chain is common to the IL-3, IL-5 and GM-CSF receptors and has been shown to signal through the JAK/Stat pathway.
There are no reviews yet.