Free Shipping in the U.S. for orders over $1000. Shop Now>>

Due to the growing problem of non-specific and cross-reactive antibodies on the market, there has been a push for more rigorous antibody validation and verification. At NeoBiotechnologies, many of our antibodies are validated for specificity using the CDI HuProt™ Human Proteome Microarray. Each microarray contains 21,000 human proteins and protein isoforms, making it the gold standard for testing antibody specificity.

Western blotting, also known as immunoblotting, is a widely used technique for detecting and quantifying specific proteins in a complex mixture and validating antibodies. NeoBiotechnologies takes care to ensure that its antibodies will perform in this common yet critical molecular biology method.
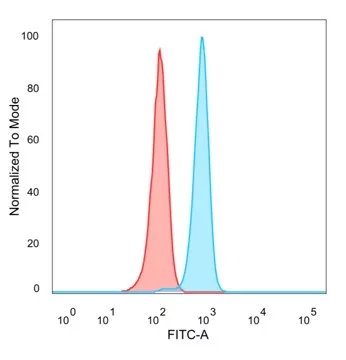
Flow cytometry is a powerful technique used for the quantitative analysis of cell populations and the detection of specific cancer and cell biomarkers. Due to the its highly sensitive nature, it is critical that antibodies used in this method are truly monospecific. NeoBiotechnologies tests all flow cytometry-validated antibodies carefully to ensure they can be used for cell surface or intracellular marker detection.
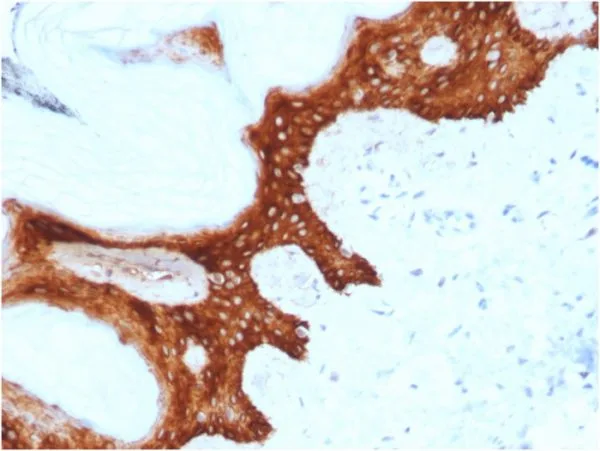
Immunohistochemistry (IHC) is a powerful technique for visualizing specific proteins in tissue sections, providing valuable insights for research and diagnostics. NeoBiotechnologies uses formalin-fixed, paraffin-embedded (FFPE) tissue samples to validate suitability of its antibodies for use in IHC applications.

Immunofluorescence (IF) is a powerful method for visualizing proteins of interest. As it is often performed under high magnitude and on proteins which may be present in small quantities, ensuring that the antibodies you use for immunofluorescence are monospecific is of paramount importance. NeoBiotechnologies’ primary antibodies undergo rigorous testing to validate them for use in immunofluorescence applications.

Tissue microarrays (TMAs) are an efficient and powerful tool for validating antibodies for immunohistochemistry, immunofluorescence, and other applications, helping to ensure specificity and rule out cross-reactivity. TMAs allow for rapid screening of sensitivity and specificity, systematic testing of off-target binding, and quantitative scoring compared to expected staining patterns. NeoBiotechnologies regularly employs tissue microarrays as part of its validation process.
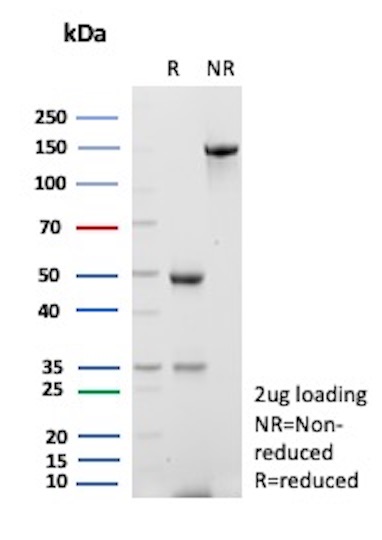
In antibody production, SDS-PAGE is an important quality control step in the production processes. SDS-PAGE functions as a robust method to assess antibodies’ purity, integrity, and molecular weight, ensuring the consistent production of premium-grade reagents tailored for research and diagnostics which perform as intended. NeoBiotechnologies routinely QCs our antibodies with SDS-PAGE in addition to our other application validation methods.

One aspect that sets NeoBiosciences apart from other antibody manufacturers is that we sequence all our hybridomas and recombinant antibodies. Our protocol yields the sequences of the paired heavy and light antibody sequences inclusive of FR1, CDR1, FR2, CDR2, FR3, CDR3, FR4, and the isotype of the antibody.