Free Shipping in the U.S. for orders over $1000. Shop Now>>

Immunofluorescence Analysis of PFA-fixed MCF-7 cells using EIF2S1 Mouse Monoclonal Antibody (PCRP-EIF2S1-1E2) followed by goat anti-mouse IgG-CF488 (green).

Immunofluorescence Analysis of PFA-fixed HeLa cells using EIF2S1 Mouse Monoclonal Antibody (PCRP-EIF2S1-1E2) followed by goat anti-mouse IgG-CF488 (green). Counterstain is phalloidin.
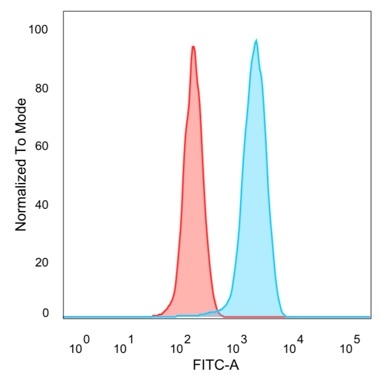
Flow cytometric analysis of PFA-fixed HeLa cells. EIF2S1 Mouse Monoclonal Antibody (PCRP-EIF2S1-1E2) followed by goat anti-mouse IgG-CF488 (blue), isotype control (red).

SDS-PAGE Analysis Purified EIF2S1 Mouse Monoclonal Antibody (PCRP-EIF2S1-1E2) Confirmation of Purity and Integrity of Antibody.

Analysis of Protein Array containing more than 19,000 full-length human proteins using EIF2S1 Mouse Monoclonal Antibody (PCRP-EIF2S1-1E2). Z- and S- Score: The Z-score represents the strength of a signal that a monoclonal antibody (MAb) (in combination with a fluorescently-tagged anti-IgG secondary antibody) produces when binding to a particular protein on the HuProtTM array. Z-scores are described in units of standard deviations (SD's) above the mean value of all signals generated on that array. If targets on HuProtTM are arranged in descending order of the Z-score, the S-score is the difference (also in units of SD's) between the Z-score. S-score therefore represents the relative target specificity of a MAb to its intended target. A MAb is considered to specific to its intended target, if the MAb has an S-score of at least 2.5. For example, if a MAb binds to protein X with a Z-score of 43 and to protein Y with a Z-score of 14, then the S-score for the binding of that MAb to protein X is equal to 29.
The initiation of protein synthesis in eukaryotic cells is regulated by interactions between protein initiation factors and RNA molecules. The eukaryotic initiation complex is composed of three subunits, designated eIF2a, eIF2band eIF2g (eukaryotic translation initiation factor 2 a, band g, respectively), all of which work in concert to form a ternary complex with GTP and tRNA in the early stages of protein synthesis. eIF2a, also known as EIF2S1 or EIF2, is a 315 amino acid subunit of the eukaryotic initiation complex that functions to bind tRNA to the 40S ribosomal subunit (in a GTP-dependent manner), thereby initiating translation. In addition, the phosphorylation state of eIF2a controls the rate of tRNA translation. When eIF2a is not phosphorylated, translation occurs at a normal rate. However, upon phosphorylation by one of several kinases, eIF2a is stabilized, thus preventing the GDP/GTP exchange reaction and slowing translation.
There are no reviews yet.