Free Shipping in the U.S. for orders over $1000. Shop Now>>
Formalin-fixed, paraffin-embedded human renal cell carcinoma stained with HSP60 Mouse Monoclonal Antibody (CPTC-HSPD1-1).

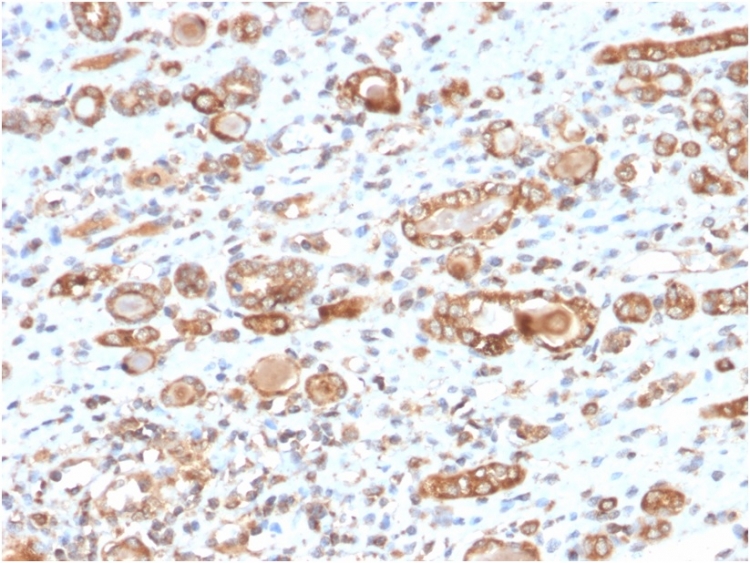
Formalin-fixed, paraffin-embedded human breast carcinoma stained with HSP60 Mouse Monoclonal Antibody (CPTC-HSPD1-1).

Immunofluorescence Analysis of human MCF-7 cells labeling HSP60 with Heat Shock Protein 60 Mouse Monoclonal Antibody (CPTC-HSPD1-1) followed by goat anti-mouse IgG-CF488 (green). Nuclei stained withRedDot.

Flow Cytometric Analysis of PFA-fixed HeLacells labeling HSP60 with HSP60 Mouse Monoclonal Antibody (CPTC-HSPD1-1) followed by goat anti-mouse IgG-CF488 (blue); isotype control (red).
The heat shock proteins (HSPs) comprise a group of highly conserved, abundantly expressed proteins with diverse functions, including the assembly and sequestering of multiprotein complexes, transportation of nascent polypeptide chains across cellular membranes, and the regulation of protein folding. The mitochondrial and cytosolic localization of HSP60, combined with its binding and catalysis of folding of newly synthesized proteins destined for the mitochondrial matrix, classify this protein as a molecular chaperone. An additional role of HSP 60 is to act as a cell surface marker for 纬/ �L T cell recognition, as well as being involved in a danger signal cascade immune response. HSP60 has been shown to influence apoptosis in tumor cells, and changes in its expression level may serve as a biomarker, as down-regulated HSP60 expression indicates a poor prognosis as well as a risk of tumor infiltration development, especially with regard to urothelial carcinomas. In ovarian cancer, decreased expression of HSP60 correlates with aggressive tumor types, while overexpression is correlated with a better patient prognosis.
There are no reviews yet.