Free Shipping in the U.S. for orders over $1000. Shop Now>>

Flow Cytometric Analysis of PFA-fixed HeLa cells. RPS6KA5 / MSK1 Mouse Monoclonal Antibody (PCRP-RPS6KA5-1A8) followed by goat anti-mouse IgG-CF488 (blue); isotype control (red).
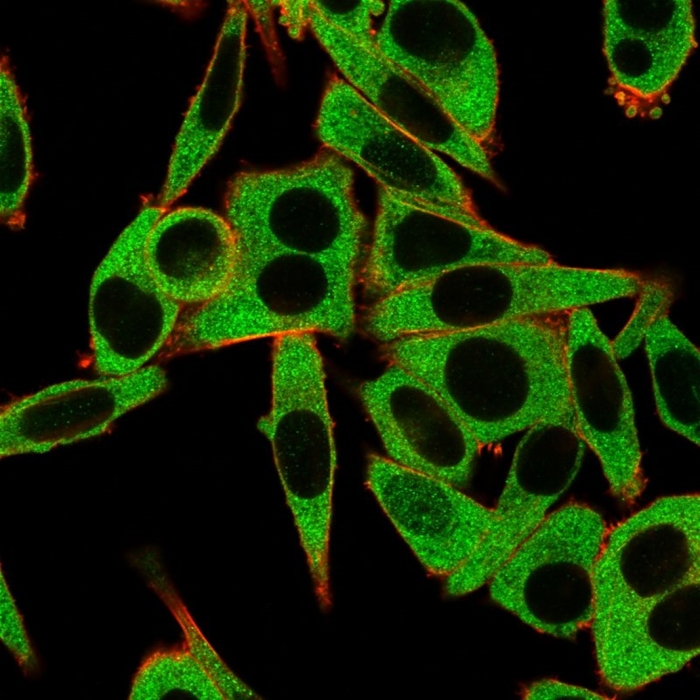
Immunofluorescence Analysis of HeLa cells using RPS6KA5 / MSK1 Mouse Monoclonal Antibody (PCRP-RPS6KA5-1A8) followed by goat anti-mouse IgG-CF488 (green). CF640A phalloidin (red).

Analysis of Protein Array containing more than 19,000 full-length human proteins using RPS6KA5 / MSK1 Mouse Monoclonal Antibody (PCRP-RPS6KA5-1A8). Z- and S- Score: The Z-score represents the strength of a signal that a monoclonal antibody (MAb) (in combination with a fluorescently-tagged anti-IgG secondary antibody) produces when binding to a particular protein on the HuProtTM array. Z-scores are described in units of standard deviations (SD's) above the mean value of all signals generated on that array. If targets on HuProtTM are arranged in descending order of the Z-score, the S-score is the difference (also in units of SD's) between the Z-score. S-score therefore represents the relative target specificity of a MAb to its intended target. A MAb is considered to specific to its intended target, if the MAb has an S-score of at least 2.5. For example, if a MAb binds to protein X with a Z-score of 43 and to protein Y with a Z-score of 14, then the S-score for the binding of that MAb to protein X is equal to 29.
The family of ribosomal S6 kinases (Rsks), designated Rsk-1, Rsk-2 and Rsk-3, have been implicated as important signaling intermediates in response to a broad range of ligand-activated receptor tyrosine kinases. A unique feature common to the three members of the Rsk family is that each possesses two non-identical complete kinase catalytic domains. A related S6 kinase, p70 S6 kinase, functions to phosphorylate the S6 protein on ribosomal 40S subunits. p70 S6 kinase b shares high sequence homology with p70 S6 kinase, except in the carboxy terminus where it contains a proline-rich domain that may be involved in SH3 domain containing protein interactions. MSK1 (also designated RLPK) is related to Rsk and p70 S6 kinase family members and is thought to be structurally similar to Rsk family members, but it may be regulated by distinct mechanisms.
There are no reviews yet.